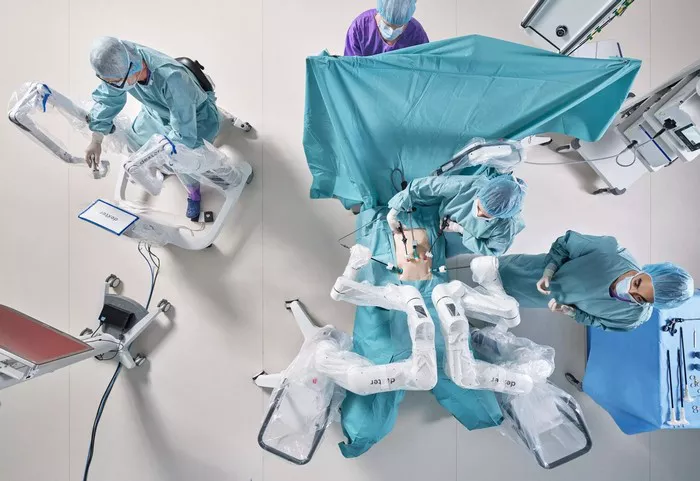
Distalmotion announced today that its Dexter surgical robot has received FDA 510(k) clearance for use in adult cholecystectomy, commonly known as gallbladder removal.
The Lausanne, Switzerland-based company already holds FDA de novo approval for Dexter in adult inguinal hernia repair, marking cholecystectomy as its second cleared indication in the U.S.
“Cholecystectomy is a major general surgery procedure with about one million cases performed annually, 60% of which occur in outpatient settings,” said Greg Roche, CEO of Distalmotion. “With approvals for both inguinal hernia repair and cholecystectomy, we are significantly expanding Dexter’s presence in the U.S. market, enabling more surgical teams and patients to benefit from robotic surgery. Our mission is to provide the right robot for the right site of care, empowering wider access to robotic technology.”
Dexter is designed as a modular, compact, and open robotic system aimed at increasing accessibility in hospitals, outpatient departments, and ambulatory surgical centers (ASCs). The robot integrates with third-party 3D imaging, energy devices, vessel sealers, and laparoscopic tools. It features fully wristed, single-use instruments that enhance dexterity, precision, and reliability while reducing the need for complex instrument reprocessing.
Distalmotion highlights that Dexter fits seamlessly into existing operating room workflows and equipment setups. Its single-use instruments simplify logistics and improve efficiency, while the surgeon’s sterile console offers direct and quick patient access, emphasizing physician control during procedures.
To date, Dexter has been used in over 2,000 cases spanning more than 30 procedure types across general, gynecological, colorectal, and urological surgeries. Distalmotion continues to grow its surgical portfolio, recently completing the pivotal HYPER study for benign hysterectomy and currently enrolling patients in a sacrocolpopexy clinical trial.
Related topics: