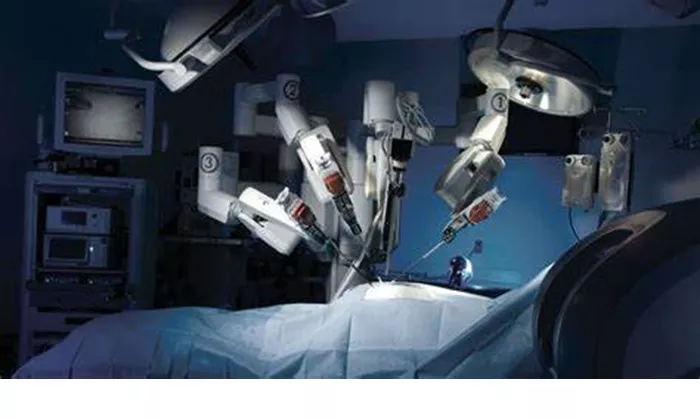
NEW BRUNSWICK, N.J. – Johnson & Johnson MedTech has achieved a major milestone with the first clinical cases completed using its OTTAVA™ Robotic Surgical System. The procedures, performed at Memorial Hermann-Texas Medical Center, mark the beginning of human trials for this next-generation surgical platform.
Pioneering Robotic Surgery
Dr. Erik Wilson of UT Health Houston successfully performed Roux-en-Y gastric bypass surgery using OTTAVA, demonstrating the system’s capabilities in complex abdominal procedures. Designed as a multi-specialty platform, OTTAVA aims to address current limitations in robotic-assisted surgery through its unified architecture and integration with J&J’s Polyphonic™ digital ecosystem.
Clinical Study Objectives
The ongoing trial will evaluate OTTAVA’s performance across various procedures, with initial focus on:
- Gastric bypass and sleeve surgeries
- Small bowel resection
- Hiatal hernia repair
Data from these cases will support J&J’s planned De Novo authorization request to the FDA for general surgery applications in the upper abdomen.
Advancing Surgical Innovation
“OTTAVA represents our commitment to transforming surgical care through clinical evidence and technological advancement,” said Hani Abouhalka, J&J MedTech Surgery Chairman. The system combines Ethicon’s surgical instrumentation expertise with robotic precision, targeting unmet needs in complex, multi-quadrant procedures.
Future of Robotic-Assisted Surgery
With IDE approval secured in late 2024, J&J continues its century-long legacy of surgical innovation through OTTAVA’s development. The company emphasizes the system’s potential to elevate standards of care across its global surgical portfolio, which spans wound closure, stapling, and digital solutions for metabolic and cardiovascular conditions.
Related Topics: